By Cat Troiano
Anemia is the condition in which a patient’s red blood cell or hemoglobin count is too low or in which the red blood cells or hemoglobin that is produced is abnormal in size or shape. Since the role of red blood cells is to transport oxygen from the lungs to the rest of the body, anemia becomes damaging when organ tissues do not receive adequate oxygen. According to the American Society of Hematology, anemia affects more than three million individuals in the United States, and it is the most common blood disorder. Understanding the different types and presentations of anemia is beneficial in choosing the proper tests that will enable physicians to treat this debilitating and potentially life-threatening condition.
Types of Anemia
There are a number of different types and causes of anemia. In order to address a patient’s anemia with the most effective treatment, it is essential to diagnose the type of anemia. Anemia is classified into three groups:
Microcytic, in which the red blood cells are smaller than normal in size
Normocytic, in which the red blood cells are normal in size, but there is an insufficient number of them
Macrocytic, in which the red blood cells are enlarged in size
Within each classification are various types of anemia. Examples of microcytic anemia include:
• Iron deficiency, which results either from blood loss or from poor absorption of dietary iron
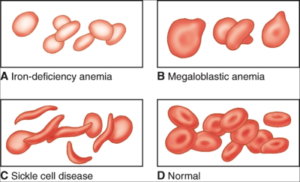
• Sickle cell anemia, which is a hereditary form of anemia that is characterized by abnormally-shaped red blood cells
• Thalassemia, which is a hereditary condition in which the hemoglobin level is too low
Examples of normocytic anemia include:
• Aplastic anemia, in which the bone marrow is unable to produce an adequate supply of all blood cells
• Hemolytic anemia, which results in the premature destruction of red blood cells before the end of their typical 120-day lifespan
• Anemia of chronic disease, such as that caused by chronic renal insufficiency in which the kidneys fail to produce enough erythropoietin, which is the hormone that aids in red blood cell production
Examples of macrocytic anemia include:
• Vitamin deficiency, such as a deficiency in vitamin B12 or in folate
• Alcoholism anemia
• Anemia caused by the use of chemotherapy agents, anti-seizure medications and antiviral drug therapies
Awareness of the different types of anemia and their causes is helpful in guiding physicians as they determine which patients should be screened for anemia.
Risk Factors and Signs of Anemia
Patients with certain risk factors should be monitored for anemia. Some of these risk factors include:
• Autoimmune diseases, such as rheumatoid arthritis
• Human immunodeficiency virus
• Gastrointestinal diseases, such as ulcerative colitis and Crohn’s disease
• Liver disease
• Kidney disease
• Hypothyroidism
• Cancer
• Pregnancy
• Heavy menstruation
• Bariatric surgical procedure
• Exposure to certain toxic chemicals, such as lead
• Travel to locations where malaria is prevalent
• Excessive aspirin or ibuprofen use
• Excessive alcohol consumption
• Being older than 65 years of age
• Family history of sickle cell anemia or other inherited forms of anemia
Some patients with mild cases of anemia may be asymptomatic, but most moderate and severe cases present with any of the following signs:
• Weakness
• Fatigue
• Dizziness
• Exercise intolerance
• Pallor
• Rapid or irregular heartbeat
• Headache
• Chest pain
• Shortness of breath
• Cold extremities
One of the first laboratory tests that reveals the presence of anemia is the routine complete blood count.
Complete Blood Count
The complete blood count (CBC) is typically ordered along with a metabolic profile as part of a routine physical or when a patient presents with signs of illness. The CBC, which is performed on a whole blood sample, measures the different components of blood, such as the various types of white blood cells, platelets and red blood cells (RBCs). The normal reference range for RBCs on a CBC is:
Men: 5 million to 6 million cells per mcL
Women: 4 million to 5 million cells per mcL
A test result that comes in lower than the reference range is indicative of anemia.
The CBC also measures hemoglobin, which is an iron-rich form of protein found in RBCs that takes up oxygen from the lungs for transport to other organs and tissues throughout the body. Each RBC has more than 200 million molecules of hemoglobin. The normal reference range for hemoglobin on a CBC is:
Men: 13.5 to 17.5 grams per deciliter
Women: 12 to 15.5 grams per deciliter
A test result that reveals the hemoglobin level to be lower than the reference range is indicative of anemia.
Hematocrit, or packed cell volume, is also measured on a CBC. The hematocrit level determines how much space the RBCs occupy within the blood. The normal reference range for hematocrit is:
Men: 41-50 percent
Women: 36-44 percent
A test result in which the hematocrit level is lower than the reference range is indicative of anemia.
Mean corpuscular volume, or MCV, is also assessed as part of the CBC, and it determines the average size of red blood cells. The normal reference range score for MCV is:
Men: 80-96
Women: 82 – 98
If a CBC result reveals that any of the aforementioned values are lower than the normal reference ranges, and if the metabolic panel reveals no evidence of chronic disease, additional tests may need to be ordered to determine the type of anemia.
B-12 Test
Vitamin B12 is needed to play a role in the production of healthy RBCs and in maintaining healthy nerve cells. This micronutrient is found only in animal-based food sources, including meat, fish, poultry, eggs and dairy products. If a patient is not taking in a sufficient amount of vitamin B12, then vitamin B12 deficiency anemia results. Alternately, if a patient’s stomach is not producing an adequate level of intrinsic factor, which is a protein that binds with vitamin B12 in the intestines to facilitate absorption, a specific type of vitamin B12 deficiency anemia known as pernicious anemia results. A patient’s vitamin B12 level can be assessed with serum from a blood sample. The normal reference range for a vitamin B12 test is:
180 to 914 ng/L
If the test result is lower than the reference range, then vitamin B12 deficiency anemia is diagnosed. Certain medications, including aspirin, contraceptive hormone drugs and anticonvulsants can cause a low vitamin B12 level. If the patient does not take any of these medications, an intrinsic factor blocking antibody test may then be performed to determine if the patient has pernicious anemia. Elevated vitamin B12 results can occur in patients with advanced liver disease, diabetes, heart failure and acquired immunodeficiency syndrome.
Folate
Folate deficiency anemia results when there is an abnormally low level of folic acid in the blood. Folic acid is another B vitamin that also plays a role in RBC production. Folic acid is consumed from leafy green vegetables, yeast and fresh fruits, and it is also found in fortified food products, such as cereals and orange juice. Since a growing fetus requires plenty of folic acid for brain and spinal cord development, pregnant women are especially at risk for folate deficiency anemia. Testing the serum from a blood sample can determine if a patient has a folate deficiency. The normal reference range for a serum folate test is:
Greater than or equal to 4.0 mcg/L
A test result that is lower than 4.0 is suggestive of folate deficiency.
Ferritin Serum
Ferritin is a type of protein that contains iron, which plays a role in healthy RBC production. The concentration of ferritin that is found in the serum of a blood sample reflects the level of iron stores in the body. The normal reference range for a ferritin serum test is:
Men: 24 to 336 mcg/L
Women: 11 to 307 mcg/L
When a test result is lower than the reference range, then iron deficiency anemia is suspected. An abnormally high result can occur in patients with certain chronic illnesses and neoplastic disease.
Reticulocyte Count
RBCs are produced in the bone marrow. Reticulocytes are immature RBCs. A reticulocyte count, which is performed on a whole blood sample, reveals the bone marrow’s level of production capability. The normal reference ranges for adults are:
Percentage of reticulocytes: 0.60 to 2.71 percent
Absolute reticulocytes: 30.4 to 110.9 X 10(9)/L
Results that are lower than the reference range can be due to heavy or chronic blood loss or hemolytic anemia. Results that exceed the reference range can be due to vitamin deficiency anemia, iron deficiency anemia, chronic kidney disease, cancer, aplastic anemia and alcoholism.
Some forms of anemia cannot be prevented and can be fatal. Others can be prevented or treated with dietary supplementation, and still others may be treated with medical procedures.
When a patient presents with potential signs of anemia and has abnormally low RBC, hemoglobin and hematocrit values on a CBC, conducting a thorough evaluation of his or her medical history and ordering the appropriate additional tests is essential in determining the type and cause of the anemia and in formulating the most effective treatment plan.
 Deer Tick
Deer Tick