As we enter Phase 2 of reopening, we begin to train our team to assist Lab Techs in Lab procedures and equipment.

By Larry Fox
As we enter Phase 2 of reopening, we begin to train our team to assist Lab Techs in Lab procedures and equipment.


By Larry Fox
We are here for all your Physician Office Laboratory needs!



Pictured above from left: Larry Fox, Lab Director, Maya Cohen and Gina DeLisa, Lab Technologists, and Cormac Barrett, VP of Lab Sales.
By Larry Fox
By Cat Troiano
Although the rate of breast cancer diagnosis has decreased over the last two decades, it is estimated that more than 300,000 women will be diagnosed with breast cancer in 2019, with more than two-thirds of these cases being invasive breast cancer. While skin cancer is the most frequently diagnosed form of cancer in women, breast cancer comes in second. When it comes to cancer-related deaths, breast cancer also comes in second, with lung cancer being the number one cause of cancer-related death in women. The projected mortality rate for women with breast cancer in 2019 is nearly 42,000. For all women and for anyone with a mother, wife, daughter or sister, these numbers are alarming. However, the mortality rate has been declining over the last 30 years, thanks in part to heightened awareness and early detection.
One of the first keys in early detection is awareness of the risk factors of breast cancer. The chances of developing breast cancer increase for individuals with the following risk factors:
Age. Women over the age of 55 account for two out of three breast cancer diagnoses, indicating that advancing age comes into play as a risk factor.
Family history. A woman’s chance of developing breast cancer doubles when a first-degree female relative, such as her mother, sister or daughter, is diagnosed.
Ethnicity. African-American women have a higher risk of developing aggressive forms of breast cancer and are typically diagnosed at a younger age. Overall, however, white women are at a slightly higher risk than African-American women for developing breast cancer, while Asian and Hispanic women have a lower risk.
Reproductive health history. Women who began menstruating prior to the age of 12 have a higher risk of developing breast cancer, as do those who go through menopause after the age of 55 and those who either never gave birth or who gave birth to their first child after the age of 30.
Prior breast cancer history. Women who have been previously diagnosed and treated for breast cancer are three to four times more likely to develop another form of breast cancer.
Prior radiation treatment. Women who receive radiation to treat a condition in the upper body, such as non-Hodgkin’s lymphoma or Hodgkin’s disease, at less than 30 years of age are at increased risk for developing breast cancer.
Breast composition. Women with non-cancerous, or benign, beast conditions as well as those with dense breast tissue have higher risks for developing breast cancer.
Chemical exposure. Women who are frequently exposed to certain chemical substances found in food, cosmetics, skin care products, lawn and gardening chemicals, plastics and even water have higher risks of developing breast cancer.
Lifestyle. Women who are overweight have a higher risk for developing breast cancer, as do women who practice unhealthy lifestyle habits, such as being sedentary, smoking, excessive alcohol consumption and consuming an unhealthy diet.
Genetics also take on a role in breast cancer risk. Up to 10 percent of breast cancer cases are believed to be linked to genetic mutations, which may be passed down from a parent.
BReast CAncer gene one (BRCA1) and BReast CAncer gene two (BRCA2) are actually genes that everyone has in their bodies. These genes carry out cellular repair for maintaining the health and function of the breasts as well as of the ovaries and other cells in the body. When either of these genes undergoes a mutation, they can no longer carry out their tasks as effectively. These ineffective genes get passed down through subsequent generations. Having one of these mutations does not seal a patient’s fate that they will develop breast cancer, but their risk does increase. It is important to remember that, while the incidence is much lower, men can also develop breast cancer, and they can develop the BRCA1 and BRCA2 mutations and pass them down to their offspring.
Just as knowing the risk factors for developing breast cancer is a crucial step toward early detection, being aware of the factors that contribute to the likelihood of carrying a genetic mutation is important. Women are more likely to have the BRCA1 and/or BRCA2 mutation(s) if:
Laboratory testing to detect the presence of these mutations should be considered for any female patient who falls into any of the above groups or carries any of the aforementioned risk factors of breast cancer. Blood tests can detect the presence of BRCA1 and BRCA2 mutations.
Positive test results confirm the presence of one of these mutations and thus place the patient in a significantly higher risk category for developing breast cancer.
Tumors release specific proteins into the bloodstream. These proteins are known as tumor markers, and blood tests to seek out the presence of these tumor markers enable pathologists to detect cancer. There are several tumor markers that are associated with breast cancer:
The normal reference ranges for these tumor marker tests are:
While tumor marker tests alone are inadequate screenings for the initial diagnosis of breast cancer, they can be valuable tests for determining the best course of treatment for a patient with breast cancer, for evaluating the efficacy of the treatment protocol and for monitoring a patient for breast cancer recurrence.
The American Cancer Society guidelines recommend that women who test positive for BRCA1 or BRCA2, or women who have any of the aforementioned risk factors for developing breast cancer, should undergo annual mammogram screenings beginning at age 30. In addition to mammography, ultrasound and/or magnetic resonance imaging of the breasts may be recommended for some women based upon their risk factors and on findings from physical examinations and laboratory tests. Women who are not considered high risk for developing breast cancer are advised to undergo annual mammogram screenings starting at age 40 to 45. All women should discuss their risk factors with their physicians to come up with the appropriate plans of preventative screenings that will provide the best chances for early detection, less aggressive treatment protocols and complete and lasting remission.
By Larry Fox
Did you know that there are numerous different types of arthritis? Arthritis is a collective term that covers more than 100 conditions characterized by longterm joint inflammation that can result in permanent joint damage. Some of the more commonly known forms include osteoarthritis, gout, rheumatoid arthritis, psoriatic arthritis and fibromyalgia. Rheumatoid arthritis, or RA, is the third most prevalent form of arthritis, affecting 1.3 million Americans. This debilitating condition affects quality of life and can reduce a patient’s lifespan. Early intervention with treatment protocols can greatly improve these factors once assessment of a patient’s risk factors and symptoms and proper testing to diagnose the condition has been performed.
RA is a chronic inflammatory condition that targets the joints. Unlike osteoarthritis, which is also known as degenerative joint disease, remains the most commonly diagnosed form of arthritis and is characterized by reduction in bone mass and an increase in susceptibility to fractures, RA affects the synovial lining of the joints. This weakens the tendons and ligaments, the connective tissues which attach muscle to bone and bone to bone, respectively, resulting in restricted joint stability and mobility, pain, and deformity as the joint capsule erodes. The damage doesn’t stop there, however. Over time, the inflammation of RA can damage other tissues in the body, including those of the lungs and heart. Unlike osteoarthritis, which results from aging and wear and tear, RA is categorized as an autoimmune disorder, which is defined as a condition in which the body’s immune cells attack the body’s own healthy tissues. Lupus, multiple sclerosis, Crohn’s disease, Guillain-Barre syndrome, Hashimoto’s thyroiditis, Graves’ disease, celiac disease and type 1 diabetes are all other examples of autoimmune disorders.
RA’s disease progression varies from patient to patient. In some, the disease advances rapidly, while in others, it progresses more slowly. Many patients experience remission periods in which their symptoms abate, and then these durations of relief end with a return of the symptoms. When this occurs, the patient is said to be experiencing a disease flareup. A few lucky patients will experience a permanent remission.
The exact cause of RA remains unknown, but several potential risk factors have been identified. These risk factors include:
Family history. Although there are patients who are diagnosed with RA without any known family history of the disease, if an individual who does have a first-degree relative, such as a parent, who has been diagnosed with RA, then that individual is at an estimated four times greater risk than someone without the family history for developing the condition.
Gender. Roughly 75 percent of individuals stricken with RA are women. This may be due in part to hormonal shifts that occur during perimenopause, as age appears to present another potential risk factor.
Age. RA can affect individuals at any age, but the condition is most prevalent in those aged 45 years and older.
Smoking and environmental hazards. Smoking appears to not only increase one’s risk for developing RA, but it may also heighten the severity of the condition. Exposure to certain hazardous materials, such as asbestos, is also suspected to increase the risk of RA development.
Obesity. Individuals who are overweight or obese may carry a greater risk for developing RA.
Again, these are potential risk factors. Since the cause of RA remains a bit of a mystery, these risk factors must be considered in addition to the patient’s symptoms before suspecting RA.
At the early stage of RA, joints in the fingers and toes are affected, presenting with swelling and discomfort. The joints maybe warm to the touch, and redness may be observed. Patients may experience stiffness in the joints upon awakening and following periods of inactivity. As RA advances, these symptoms gradually extend to the wrists, ankles, elbows, knees, shoulders and hips. One of the factors that typically differentiates RA from osteoarthritis us that in RA patients, affected joints are usually symmetrical, meaning that the same joint on both sides of the body are affected. For example, a patient may be experiencing discomfort in the left hand as well as in the right hand.
While localized joint pain and inflammation is the initial complaint, additional symptoms of RA may include:
When four or more joints are affected, a patient complains of experiencing these symptoms for a duration of six months or longer or has any of the risk factors mentioned above in addition to their presenting symptoms, the diagnostic process should include testing for RA.
Once an evaluation of a patient’s symptoms and history has been performed, laboratory tests are ordered to determine whether or not the patient’s arthritis is specifically RA. This determination can often be made through a combination of two laboratory tests: the rheumatoid factor screening test and the anti-cyclic citrullinated peptide test.
Rheumatoid factor (RF) is an immunoglobulin-M protein autoantibody which is present in roughly 80 percent of patients who have RA, indicating an inflammatory state and autoimmune condition. The normal reference range for an RF test is less than 20 u/ml. However, there are other conditions that can yield an elevated RF level, including other autoimmune disorders, cancer, diabetes, some chronic infections, some vaccines and even the normal aging process. It is also important to note that approximately 20 percent of patients who have RA will have normal RF levels. Therefore, it is important to order an anti-CCP test in conjunction with the RF test.
Anti-cyclic citrullinated peptide (anti-CCP) is another autoantibody that is present in 60 to 70 percent of RA patients. When a patient’s RF and anti-CCP tests both yield positive results, the diagnosis is known as seropositive RA. The normal reference range for an anti-CCP test is less than 20 u/ml.
The remaining percentage of symptomatic patients whose RF and anti-CCP tests both yield negative results are diagnosed with seronegative RA. These patients may yield results on other laboratory screenings that are indicative of inflammation.
Although not specific to RA detection, the erythrocyte sedimentation rate (ESR) test and the C-reactive protein tests, both of which indicate the presence of inflammation, can be helpful in diagnosing RA in that elevated levels in these two tests are typical results in RA patients, but not in patients with osteoarthritis. These tests can also be useful in monitoring the condition’s level of activity as the disease progresses and when patients experience RA flareups.
The ESR test evaluated the rate at which erythrocytes, or red blood cells, settle into the bottom of the test tube apart from the plasma portion of the blood. When there is an increased level of acute phase reactant proteins, such as C-reactive protein, the erythrocytes drop to the bottom of the tube at a faster rate. A higher than normal level of C-reactive protein, and thus a higher sedimentation rate, occur in the presence of inflammation. The normal reference ranges for these two tests are:
ESR
Men less than 50 years of age: 0 to 15 mm/hr. to 0 to 20 mm/hr.
Women less than 50 years of age: 0 to 20 mm/hr. to 0 to 30 mm/hr.
Normal results tend to be higher in patients who are 50 years of age and older.
C-reactive protein
Less than 1.0
The ESR and C-reactive protein tests alone cannot be used to attain a definitive diagnosis of RA, however, since other factors, such as advanced age, the presence of infection or obesity, can cause elevated results in both tests.
If the above tests yield results that do not facilitate a confident diagnosis of RA, then diagnostic imaging tests, such as radiographs and magnetic resonance imaging, will come into play to visualize the condition of the affected joints, allowing physicians to rule out other causes of discomfort, make the diagnosis and assess the severity of the disease. These imaging tests, along with the ESR and C-reactive protein tests, may also be utilized to monitor the disease progression. The RF and anti-CCP tests are not particularly helpful in monitoring disease progression in that a patient who had elevated levels at the time of diagnosis will continue to have elevated levels, even during periods of disease remission. Complete blood counts and metabolic profiles should be ordered periodically to monitor for potential RA complications as well as any side effects that can result from drug therapies used to treat the condition.
As RA progresses, complications can come about. Some potential complications of RA include:
Frequent evaluations and longterm monitoring of patients with RA are imperative. Periodic laboratory and imaging tests are essential to track the disease’s progression as well as to identify complications and to treat accordingly.
There is no cure for RA. Treatment goals are to reduce pain and inflammation and to retard the progression of the disease. Early treatment with disease-modifying antirheumatic drugs can slow the progression of a patient’s RA and increase the chances for remission. Other treatment options include:
When other methods of treatment are ineffective, surgical intervention to repair or to remove and replace damaged joints may be considered.
Patients who are referred to a board-certified rheumatologist to begin treatment at the earliest possible stage of the disease, before extensive joint damage has been sustained, have the best chance at living a full and active life.
By Larry Fox
by Cat Troiano
Hypoadrenocorticism, more commonly known as Cushing’s syndrome, is an endocrine disorder that is characterized by hypercortisolism, which is an abnormally high level of the hormone cortisol in the blood. Between 10 and 15 million individuals in the United States are diagnosed with this condition each year. Diagnosing Cushing’s syndrome can be challenging due to the fact that some of its presenting symptoms mimic those of other conditions as well as to the fact that there are a number of potential causes of hypercortisolism.
Often coined the stress hormone, cortisol is produced in the adrenal cortex, which is the outer layer of the adrenal glands, which are situated just superior to the kidneys. Cortisol is secreted in response to stressful conditions to prepare the body to function optimally during such times. Similarly, the slow-acting hormone is also released in response to imposed physical demands during exercise. Regulatory functions of cortisol include:
Multiple glands are responsible for the normal production of cortisol. First, the hypothalamus produces corticotropin-releasing hormone. This hormone stimulates the pituitary gland, which is a pea-sized gland that sits at the base of the brain, just below the hypothalamus. The pituitary gland produces another hormone, called adrenocorticotropic hormone (ACTH) in addition to other hormones, including growth hormone and vasopressin. ACTH is the hormone that stimulates cortisol production in the adrenal glands.
Both genders can develop Cushing’s syndrome, but it is most commonly diagnosed in women between the ages of 30 and 50. Longterm uncontrolled hypertension and longterm high blood glucose levels in diabetics are risk factors for developing Cushing’s syndrome. There are several causes of high cortisol levels:
Pituitary adenoma, which is a benign tumor on the pituitary gland, promotes an overstimulation of ACTH hormone, which in turn stimulates excess cortisol production from the adrenal gland. The specific name for this condition is Cushing’s disease, and it accounts for more than half of all hypoadrenocorticism cases.
Glucocorticoid drug use over the longterm, such in the treatment of such conditions as asthma and autoimmune disorders as well as injectable formulations used to relieve joint pain and administration of such drugs following organ transplants, is another common cause of hypoadrenocorticism. This exogenous form is referred to as iatrogenic Cushing’s syndrome.
Adrenal tumors, which may be benign or malignant, develop on the adrenal gland and prompt cortisol production. Cancerous adrenocortical carcinomas are the least common cause of hypoadrenocorticism.
Ectopic ACTH-producing tumors overproduce ACTH, which in turn triggers the production of cortisol by the adrenal gland. Such tumors are not located in the pituitary or adrenal glands. Instead, they develop in other parts of the body, such as in a lung, or other glands, such as the thyroid or the pancreas. This form of hypoadrenocorticism is referred to as ectopic Cushing’s syndrome)
While most cases of hypoadrenocorticism are not hereditary, there are incidences of familial Cushing’s syndrome in which younger individuals carry a genetic mutation that predisposes them to develop cortisol-producing tumors on one or more glands of the endocrine system.
The list of signs and symptoms that present with Cushing’s syndrome is lengthy. These indicators include:
Cushing’s syndrome is usually curable. However, when left untreated, complications ensue, some of which can have fatal repercussions. Complications of longterm hypercortisolism include:
Such complications emphasize the importance of testing all individuals who present with a physical appearance that may be indicative of hypoadrenocorticism in order to make a diagnosis and design a treatment plan. Patients who have hypoadrenocorticism may generate abnormal findings on general laboratory screenings, including elevated white blood cells and neutrophils on a complete blood count, hypokalemia on a metabolic profile or impaired function on a glucose tolerance test. Specific tests for Cushing’s syndrome must then be performed to make a diagnosis.
All tests for Cushing’s syndrome have limitations in that a number of factors can generate false-positive results. One such factor is the condition known as pseudo-Cushing’s. Pseudo-Cushing’s is a state in which an individual produces abnormally high levels of cortisol, but often does not present with the aforementioned physical attributes that are associated with Cushing syndrome. The causes of these elevations differ from those with true Cushing’s syndrome, and they include:
Pseudo-Cushing’s can affect the results of many Cushing’s tests, as can the use of certain drugs, such as oral contraceptives, and other medical conditions, such as hyperthyroidism.
To complicate matters further, as mentioned above, there are multiple potential causes of Cushing’s syndrome, and it is crucial to determine not only whether or not a patient actually has Cushing’s syndrome, but to determine the cause of their condition as well. Fortunately, there are multiple tests for diagnosing Cushing’s syndrome, and a testing sequence is the recommended method for attainting a diagnosis. First, one or more initial tests are performed to detect abnormal cortisol levels. Once abnormal levels are confirmed, then secondary tests are performed to determine the cause of the abnormal results.
24-Hour Urinary Free-Cortisol Test
The 24-hour urinary free-cortisol test provides a measurement of how much cortisol a patient’s body produces over the course of a 24-hour duration. This is typically the initial test performed to determine if a patient has hypoadrenocorticism. The normal reference range for the 24-hour urinary free-cortisol test is less than 40 to 50 ug/d. A result that exceeds this range is suggestive of Cushing’s syndrome. However, patients with early or mild cases may generate normal results on the 24-hour urinary free-cortisol test.
Low-Dose Dexamethasone Suppression Test
The low-dose dexamethasone suppression test evaluates how the cortisol-producing adrenal glands respond to the ACTH hormone. The testing procedure requires that the patient receives one milligram of dexamethasone, which is a synthetic glucocorticoid drug, in the evening, followed by a blood draw on the next morning. Normally, the dose of dexamethasone should suppress the production of ACTH during the overnight hours, resulting in a low morning serum cortisol level. However, if a patient does indeed have Cushing’s syndrome, then this normal response does not kick in, and thus their morning cortisol level will be abnormally high. A test result of 1.8 ug/dL or greater is a positive result. It is important to note that patients who slept poorly, who are currently under excessive emotional or physical stress or those who are obese may generate false-positive results on this test.
Late-Night Salivary Cortisol Test
The late-night salivary cortisol test measures cortisol levels in 1 mL of a patient’s saliva over the span of one hour, typically between 11 p.m. and midnight when cortisol production is low in patients without Cushing’s syndrome. A result that exceeds the normal reference range of 0.10 to 0.15 ug/dL is suggestive of Cushing’s syndrome.
If any of these initial tests generate positive results, repeat testing should be ordered to rule out false-positive results before proceeding to the secondary phase of testing. The secondary tests are performed to discern the cause of hypercortisolism.
Corticotropin-Releasing Hormone Stimulation Test (CRH stimulation test)
This test measures the level of cortisol in the blood in response to the injection of a synthetic CRH. An initial blood sample is taken prior to the injection, and then additional blood samples are collected at intervals during the one to three hours that follow the injection. The result of this test helps the physician to determine the cause of a patient’s abnormal ACTH and cortisol secretion levels. As mentioned above, Cushing’s syndrome can result from pituitary adenomas or from ectopic tumors. The results of the CRH stimulation test can distinguish between pituitary adenoma, which will yield peaks in ACTH and cortisol levels on the CRH stimulation test, and ectopic Cushing’s syndrome, which will not exhibit these elevations.
High-Dose Dexamethasone Suppression Test
This test can also help to pinpoint the cause of a patient’s Cushing’s syndrome. The procedure for administering the high-dose dexamethasone test differs from that of the low-dose dexamethasone suppression test only in that the patient must take a higher dose – 8 mg – of dexamethasone. At this dose, patients whose test results reveal low morning serum cortisol levels may have pituitary tumors, whereas those with high morning serum cortisol levels may have adrenal tumors or ACTH-producing tumors elsewhere in the body, such as in the lungs.
Dexamethasone-Suppressed Corticotropin-Releasing Hormone Test
This test combines the dexamethasone suppression test with the CRH stimulation test with the goal of ruling out a pseudo-Cushing diagnosis. Patients with Cushing’s syndrome will have elevated cortisol levels, whereas those with pseudo-Cushing will not have elevated cortisol levels as a result of this test.
Petrosal Sinus Sampling Test
This invasive testing procedure is relegated only to patients for which a Cushing’s syndrome diagnosis has been confirmed. ACTH normally drains from the pituitary gland into the inferior petrosal sinus veins. For the petrosal sinus sampling test, blood samples are taken from these veins through a catheter. One sample is taken before the administration of corticotropin-releasing hormone, and then additional samples are taken at intervals over a short time span that follows the injection. The ACTH levels that are revealed in these samples are compared to those of samples that are taken from a peripheral vein. Higher ACTH levels in the samples taken from the petrosal sinus veins are indicative of pituitary adenoma. If the ACTH level in these samples is similar to that of the samples taken from the peripheral vein, then such a result is suggestive of ectopic Cushing’s syndrome.
Once a diagnosis of Cushing’s syndrome has been achieved, diagnostic imaging tests, such as magnetic resonance imaging, computed tomography and ultrasound, may be ordered to evaluate the patient’s lungs, abdominal organs and pituitary and adrenal glands for tumors, hyperplasia and other abnormalities.
The treatment for hypoadrenocorticism depends upon the cause of the hypercortisolism. In the case of iatrogenic Cushing’s syndrome, a patient’s use of glucocorticoid drugs will likely need to be weaned down to the most minimal dose that can be sustained to effectively manage the condition for which he/she is taking the medication. Surgery to remove pituitary adenomas is the effective treatment method for patients with Cushing’s disease, and surgical removal is also performed when ACTH-producing tumors are diagnosed elsewhere in the body. Other treatment options for Cushing syndrome include radiation, chemotherapy and drug therapy to reduce the production of cortisol. Patients who are suspected to have Cushing’s syndrome are usually referred to an endocrinologist for testing, treatment and management plans.
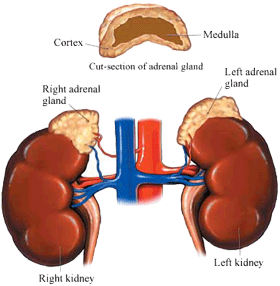
Thanks to Johns Hopkins for the image.